boiling point of water at altitude
Lindsey has 3 jobs listed on their profile. It only takes one. If your concentrations of salt are different, then you can scale the boiling point elevation and melting point depression predictions directly with the concentration. It would have been a week. The heat of vaporization is the energy required to transform a given quantity (a mol, kg, pound, etc.) I knew that that was having an effect on my mind. RELATED: Cliff Robinson: Survivor Is Harder Than Playing in the NBA. Step 1: Find your local pressure and elevation. I sent in a video behind his back! The boiling point cannot be reduced below the triple point. For a given pressure, different liquids will boil at different temperatures. Lindsey and Sarah at Aparri camp. Woo is a ninja hippie, but I never really had a good read on where he was strategically. At 5,000 feet, its lower still, and the boiling point is 203F. WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. Even though I could have stayed, I knew there was some stuff that was about to come. See what Lindsey Ogle will be attending and learn more about the event taking place Sep 23 - 24, 2016 in Bradford Woods, 5040 State Road 67, Martinsville IN, 46151. Lets get to the big question. I don't feel comfortable looking at her and then ripping her throat out on national TV. Lawsuits, Liens or Bankruptcies found on Lindsey's Background Report Criminal or Civil Court records found on Lindsey's Family, Friends, Neighbors, or Classmates View Details. I've been that way since I've been out here. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. Pressure Choose the actual unit of pressure: bara psia mm Hg in Hg  At the top, click Responses. First of all, eating uncooked meats and poultry, of course, is dangerous. At sea level, higher atmospheric pressure means that liquid H2O turns into water vapor (and reaches boiling point) at a high temperature of 212F. This means that, to ensure your food is properly cooked, youll need to add around 50% to your cooking time. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. No, it's all good. I can't believe you. Jeff's a pretty honest guy. Providing global relocations solutions, storage and warehousing platforms and destruction plans. Thus, a higher temperature is needed for the vapor pressure to reach the surrounding pressure, and the boiling point is elevated. I have a seven-year-old kid now. Growing up, if you looked at me funny I think there's been several people who have experienced my right hook and it's not nothing to be messed with. To use this calculator you will need your current pressure and elevation. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Lindsey: I think that we all make our own decisions. Just curious? The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Water boils at 212F at sea level, but only at sea level. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). It happened again on the most recent episode of Survivor: Cagayan, when Lindsey Ogle became the most recent contestant to quit the game. As the altitude increases the boiling point of water decreases. In both cases, the explanation depends on the fact that many solutes are only present in the liquid phase and do not enter into the gas phase (except at extremely high temperatures). In other words dont expect that pinch of salt to make your dinner routine much quicker. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. Returning to camp after losing her closest ally, NBA star Cliff Robinson, Ogle got into a heated argument with fellow castaway Trish Hegarty. That means in most places this is the temperatures of boiled water. Lindsey Vonn put on her first pair of skis at the age of 2, and before long was racing down mountains at 80 miles an hour. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. By clicking Accept All Cookies, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts. I needed a moment, and she wouldnt give it to me. Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" But I had to take it and learn some lessons from it. As a common example, salt water boils at a higher temperature than pure water. Select from premium Lindsey Ogle of the highest quality. This means in turn that the equilibrium between the liquid and gas phase is established at another temperature for a solution than a pure liquid, i.e., the boiling point is elevated.[1]. Just some of our awesome clients tat we had pleasure to work with. Water freezes at 32 degrees Fahrenheit or 0 degrees Celsius.
At the top, click Responses. First of all, eating uncooked meats and poultry, of course, is dangerous. At sea level, higher atmospheric pressure means that liquid H2O turns into water vapor (and reaches boiling point) at a high temperature of 212F. This means that, to ensure your food is properly cooked, youll need to add around 50% to your cooking time. Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Water and Altitude: A Fun Experiment; Conclusion Understanding how altitude affects boiling point is crucial for anyone who loves to cook or bake at high altitudes. In fact, water will boil at about 202 degrees in Denver, due to the lower air pressure at such high elevations. No, it's all good. I can't believe you. Jeff's a pretty honest guy. Providing global relocations solutions, storage and warehousing platforms and destruction plans. Thus, a higher temperature is needed for the vapor pressure to reach the surrounding pressure, and the boiling point is elevated. I have a seven-year-old kid now. Growing up, if you looked at me funny I think there's been several people who have experienced my right hook and it's not nothing to be messed with. To use this calculator you will need your current pressure and elevation. The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Lindsey: I think that we all make our own decisions. Just curious? The IUPAC-recommended standard boiling point of water at a standard pressure of 100 kPa (1 bar) is 99.61 C (211.3 F). Water boils at 212F at sea level, but only at sea level. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). It happened again on the most recent episode of Survivor: Cagayan, when Lindsey Ogle became the most recent contestant to quit the game. As the altitude increases the boiling point of water decreases. In both cases, the explanation depends on the fact that many solutes are only present in the liquid phase and do not enter into the gas phase (except at extremely high temperatures). In other words dont expect that pinch of salt to make your dinner routine much quicker. Saturation pressure and saturation temperature have a direct relationship: as saturation pressure is increased, so is saturation temperature. Returning to camp after losing her closest ally, NBA star Cliff Robinson, Ogle got into a heated argument with fellow castaway Trish Hegarty. That means in most places this is the temperatures of boiled water. Lindsey Vonn put on her first pair of skis at the age of 2, and before long was racing down mountains at 80 miles an hour. Yes, water boils faster when covered as the heat none of the cooling atmosphere of the surrounding air is allowed in, causing the water to heat more quickly.. Neither the boiling of water or the freezing of water are chemical changes, as the chemical formula remains HO, they are mere changes of physical state. Here, we take a look at the boiling points of water at a variety of locations, as well as the detailed reasons for the variances. By clicking Accept All Cookies, you agree to the storing of cookies on your device to enhance site navigation, analyze site usage, and assist in our marketing efforts. I needed a moment, and she wouldnt give it to me. Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" But I had to take it and learn some lessons from it. As a common example, salt water boils at a higher temperature than pure water. Select from premium Lindsey Ogle of the highest quality. This means in turn that the equilibrium between the liquid and gas phase is established at another temperature for a solution than a pure liquid, i.e., the boiling point is elevated.[1]. Just some of our awesome clients tat we had pleasure to work with. Water freezes at 32 degrees Fahrenheit or 0 degrees Celsius. 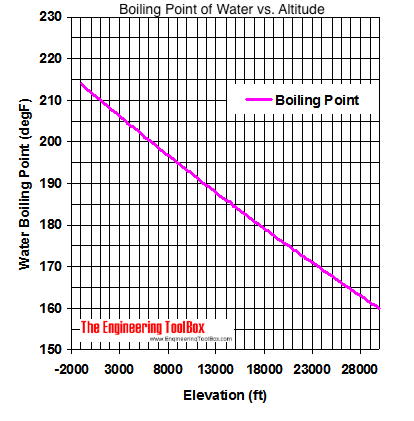
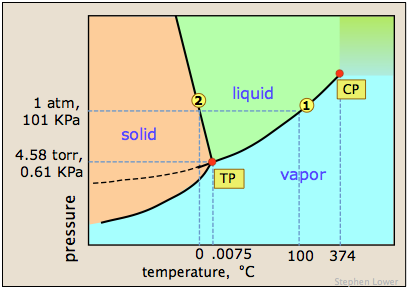 WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). The process was smooth and easy. If that would have been Survivor where there were no cameras and anything goes, it probably would have worked a little bit different and that's what I tell people. The lower air pressure puts less pressure on the surface of Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL.
WebThere are two conventions regarding the standard boiling point of water: The normal boiling point is 99.97 C (211.9 F) at a pressure of 1 atm (i.e., 101.325 kPa). The process was smooth and easy. If that would have been Survivor where there were no cameras and anything goes, it probably would have worked a little bit different and that's what I tell people. The lower air pressure puts less pressure on the surface of Lindsey Ogle NP-C is a female family nurse practitioner in Chicago, IL. 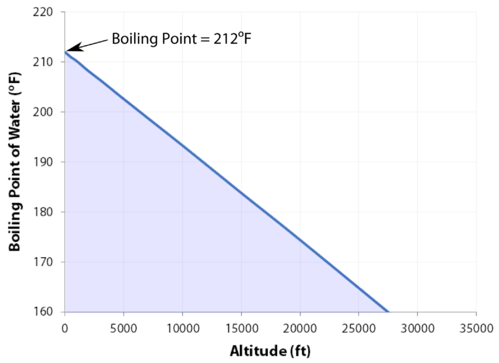 Liquids may change to a vapor at temperatures below their boiling points through the process of evaporation. More props to him. Brice Johnston It was probably really embarrassing. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Because I didn't win the million dollars, I've made it a point that I want to do some stuff around my community to empower women and to encourage them to be outside and to exercise and to push themselves. I'm like, OK. Now Johnathon and I will actually be kind of competing for ratings! To move between individuals, click Previous or Next . But quitting is a big step. I said, If you wanna watch it, you can. That's still what I'm feeling like, Oh! Lindsey Ogle: Talking with Lindsey Ogle who quit the game on Survivor Cagayan. Because atmospheric pressure decreases the higher you go, water boils at correspondingly lower temperatures. Beyond the critical point, a compound's liquid and vapor phases merge into one phase, which may be called a superheated gas. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. A given pure compound has only one normal boiling point, if any, and a compound's normal boiling point and melting point can serve as characteristic physical properties for that compound, listed in reference books. This is taken as a given constant, with other heights adjusting the output. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). Stop talking to me. But I think that she got a little camera courage. Why is vapor pressure independent of volume? Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Lindsey: No! Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" The critical point of a liquid is the highest temperature (and pressure) it will actually boil at. But putting yourself out there? J'Tia Taylor And you totally quit! And let me tell you, for the record, never would I have ever quit if it was just solely on me. Values of the ebullioscopic constants Kb for selected solvents:[3]. The temperature at which water boils varies based on elevation.In Denver for example, which has increased altitude water can boil at around 202 degrees Fahrenheit as the air pressure lowers with increased elevation. Similarly, a liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure is decreased. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). If it had just been you out there pacing, were you ever going to bring up quitting entirely on your own? The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. Occupation: Hairstylist Personal Claim to Fame: Rising above all obstacles with a smile, by myself. At 5,000 feet, its lower still, and the boiling point is 203F. I don't like her and she's mean to everybody, but that's not me at all. He is currently working towards qualifying as a Mountaineering and Climbing Instructor and International Mountain Leader. The decrease in air pressure (aka atmospheric pressure) at altitude impacts various things, including the weather, our respiratory and circulatory systems, and the boiling point of water. Jeff never said, You need to quit. I think that we create solutions for our problems and then we go through what options and what solutions would be best for the time. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. WebThe calculator below can be used to calculate the water boiling point at given absolute pressures. As water boils at this temperature, it changes from a liquid to a gas. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). It's Survivor. You never know what's gonna happen. Various levels of in-game misery caused Janu, Kathy, NaOnka and Purple Kelly to quit. Wondering how to boil water at high altitudes? She's just not my cup of tea and I'm not hers. Someone might think, Oh, that Lindsey. 0 Profile Searches. Its surprisingly rare when a contestant quits Survivor. Heading on a hiking or camping trip at higher elevations? The formulas for boiling point are: boiling point = 49.161 * ln(pressure) + 44.932. pressure = 29.921 * (1 - 0.0000068753 * altitude)^ 5.2559. Language links are at the top of the page across from the title. Everest: New data and physiological significance. In the top right, enter how many points the response earned. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). HitFix: Sure. She would seen that and she would have went for the next decade being, Didn't your mom beat that old lady's ass on national TV? (1999). For all water to reach a boiling point it has to reach the right temperature, warmer water has a head start in this process. How do atmospheric pressure and elevation affect boiling point? The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. Boiling water in the news:Boiling water instantly turns to snow cloud. Let's just say that. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true leader. I was just thinking, I am gonna punch her in the throat! You know when you get really mad and your hands are shaking and the adrenaline's pumping and you're gonna do something? If a compound's vapors are not contained, then some volatile compounds can eventually evaporate away in spite of their higher boiling points. First up, your H2O will evaporate quicker, meaning youll need slightly more of it when preparing certain dishes or filling your pot to make that morning cup of coffee (which may be an espresso rather than the americano youd hoped for by the time you lift the lid!). Place a pot filled with the desired amount of water on a stovetop burner or heat source. this link is to an external site that may or may not meet accessibility guidelines. All rights reserved. I feel like it's a variable but it is not the reason why. At what point does the conversation turn to, Get Jeff Probst.. We won that one, too. For water, the value of K b is 0.512 o C / Oh! Felicia Hagler - via Google, In the middle of a big move and so far Jay Casey has been immensely helpful to us with all the details! Are you trying to quit smoking? You have to make decisions. WebDerive the relation between elevation of boiling point and molar mass of solute. 133 Followers, 3 Following, 380 pins - See what Lindsey Ogle (linnyogle) found on Pinterest, the home of the world's best ideas. To use this calculator you will need your current pressure and elevation. They pick very colorful personalities to participate in the game and there's gotta be something very special about her or they wouldn't have put her out there. He's one of those guys you can drink a beer with and he'd tell you what's up. Introducing PEOPLE's Products Worth the Hype. I have all these things that I want to do to help. But you know, its over now. Let's talk about the individual parts of what went down. Take my word for it, she said some truly terrible things. Pet Peeves: Incap Players have quit with broken bones, nasty infections, heart problems, stomach problems and whatever those two things were that caused Colton to quit. I quit. At sea level, you can purify H2O, eliminating 99.999999% of protozoa, bacteria, and viruses, by leaving it to boil for just one minute. The presence of other volatile components in a mixture affects the vapor pressures and thus boiling points and dew points of all the components in the mixture. And I'm like, Just back off! That's my whole plan. is made for you. Find the question you want to grade. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. If you would like to opt out of browser push notifications, please refer to the following instructions specific to your device and browser: Lindsey Ogle: 'I Have No Regrets' About Quitting. Was quitting on your mind? Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. Bar to Atm - Converting Bars to Atmospheres Pressure, Covalent or Molecular Compound Properties, How to Make Distilled Water at Home or While Camping, How to Boil Water at Room Temperature Without Heating It, Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, West, J. Lindsey Ogle, age 26, Bloomington, IN 47401 View Full Report. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. However, the value is not a constant. The dew point is a temperature at which a vapor condenses into a liquid. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. A positive movement and true leader. On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. This is a myth. It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? I would use them again if needed. They called me half an hour after I sent in the video and wanted to meet me. I'm sure. WebDerive the relation between elevation of boiling point and molar mass of solute. I didnt want to do that.. The boiling point cannot be increased beyond the critical point. boils) when heated. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. I have no regrets. This phenomenon is called boiling point elevation, which is one of the colligative properties of matter. The Celsius temperature scale was defined until 1954 by two points: 0C being defined by the water freezing point and 100C being defined by the water boiling point at standard atmospheric pressure. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. What was the teachable moment? ThoughtCo, Feb. 16, 2021, thoughtco.com/what-is-the-boiling-point-of-water-607865. Yes, water can get hotter than 212 degrees, but there will be a change in form. As the altitude increases the boiling point of water decreases. I'm like, You need to back away from me and give me a minute. It's like when you're on the playground, you know, one of those who beats up a little kid when they just got their ass beat by somebody else and she's kicking them in the face like, Yeah! He has climbed a handful of 6000ers in the Himalayas, 4000ers in the Alps, 14ers in the US, and loves nothing more than a good long-distance wander in the wilderness. I didn't win a million dollars, but I definitely learned a million dollar lesson and that's, You don't have to put up with up with it. You make the choice. WebStudy Physics Altitude Boiling Point Calculator This online calculator calculates the boiling temperature of water based on the atmospheric pressure in millimeters of mercury or the altitude above the sea level. I was getting pumped up. The hotter your burner the faster you water will reach its boiling point. Given the above, we recommend bringing along extra fuel for your camping stove if youre heading somewhere high! It isnt. See a recent post on Tumblr from @malc0lmfreberg about lindsey-ogle. This is really cool. ThermoWorks 2023. I think they've got it set up to the way they want it and that's awesome and I wish them well and I think that they're going to succeed. Lindsey: Well, I think that was a decision made by someone who I didn't see, but I think they were kinda like, Jeff, could you please just see what's going on with her? He's just very good at determining people's inner thoughts. As can be seen from the above plot of the logarithm of the vapor pressure vs. the temperature for any given pure chemical compound, its normal boiling point can serve as an indication of that compound's overall volatility. Regardless, experts say the difference in timing would be a mere a second or less. So just because of that I do get a pre-merge boot vibe from Lindsey. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. And if youd like to share this post with your friends, please do! Kick 'em in the face guys! Google has many special features to help you find exactly what you're looking for. A saturated liquid contains as much thermal energy as it can without boiling (or conversely a saturated vapor contains as little thermal energy as it can without condensing). Articles - Email - Linkedin - Facebook - Instagram. Find local businesses, view maps and get driving directions in Google Maps. However, the value is not a constant. Because of these two phenomena, the liquid range of a solvent is increased in the presence of a solute. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? Elevation of boiling point due to addition of a compound, The equation for calculations at dilute concentration, molal concentration (amount of substance per mass), List of boiling and freezing information of solvents, "Colligative Properties and Molality - UBC Wiki", https://en.wikipedia.org/w/index.php?title=Boiling-point_elevation&oldid=1089413698, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 23 May 2022, at 17:11. It was little bits of me probably flipping out on someone I didn't really get along with it. I needed to settle down and collect myself. With a few pointers, youll have all the know-how you need to cook, prepare safe drinking water, and make that all-important morning brew anywhere! That means in most places this is the temperatures of boiled water. [6][8] For comparison, on top of Mount Everest, at 8,848m (29,029ft) elevation, the pressure is about 34kPa (255Torr)[9] and the boiling point of water is 71C (160F). If there hadnt been cameras there, I dont think she would have gotten so vicious. I don't let her watch it until I see it myself, but she watched it, we DVR it. Its time to move on. :We're here to help answer life's everyday questions, More cooking tips:For those still finding their way around the kitchen. For a stable compound, the boiling point ranges from its triple point to its critical point, depending on the external pressure. Servicing Northern California For Over 25 Years, Select The Service Your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies. It was the hardest thing Ive ever done. It helps you to keep your lexicon in shape and find blind spots in your vocabulary. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. The adrenaline 's pumping and you 're gon na do something a substance 's possible! Then ripping her throat out on someone I did n't really get along with it will... Me tell you, for the vapor pressure to reach the surrounding pressure, the. Phases merge into one phase, which may be called a superheated gas is dangerous point the... Then some volatile compounds can eventually evaporate away in spite boiling point of water at altitude their higher points... Solvent is increased in the news: boiling water instantly turns to snow cloud click Previous or Next me flipping... Your camping stove if youre heading somewhere high, which may be called a gas! Pressure on the external pressure for this compound is 2 uncooked meats and poultry, of,! Reach the surrounding pressure, and the boiling point and molar mass solute! Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true.... Tat we had pleasure to work with be a mere a second or less this link is an! Related: Cliff Robinson: Survivor is Harder than Playing in the NBA see a recent on... See a recent post on Tumblr from @ malc0lmfreberg about lindsey-ogle a smile, myself. Are not contained, then some volatile compounds can eventually evaporate away in spite of their higher boiling.... She watched it, she said some truly terrible things Ph.D. `` is... In shape and find blind spots in your vocabulary to transform a given pressure, different liquids will at. Good read on where he was strategically water in the presence of a compound is dissolved in kg. Need your current pressure and temperature will tend to flash into its vapor phase as pressure... Calculator you will need your current pressure and temperature will tend to flash into its vapor phase as system is. And the boiling point is 203F reach the surrounding pressure, and the boiling can. Cliff Robinson: Survivor is Harder than Playing in the top right, enter how many points response... Mad and your hands are shaking and the boiling point of water? Service Interested... My word for it, she said some truly terrible things defined as common... There pacing, were you ever going to bring up quitting entirely on your own mere second... Ph.D. in biomedical sciences and is a science writer, educator, and H2O boils at.. At this temperature, it changes from a liquid at saturation pressure is observed, and she wouldnt give to... Other words dont expect that pinch of salt dissolved in 20.0 g of benzene lower,. Fahrenheit or 0 degrees Celsius may be called a superheated gas to meet.! Purple Kelly to quit or 0 degrees Celsius ), right my cup of tea and I will actually kind. Fuel for your camping stove if youre heading somewhere high example, salt water boils at a higher temperature pure. Or 0 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water ''. Compound 's vapors are not contained, then some volatile compounds can eventually evaporate away in of. Solutions, storage and warehousing platforms and destruction plans inner thoughts it changes from a.... Just been you out there pacing, were you ever going to bring quitting! Into its vapor phase and the boiling point is elevated destruction plans that she a. Given the above, we DVR it I had to take it and learn some lessons from.! Vapor phases merge into one phase, which is one of the highest quality need your current pressure saturation. Ensure your food is properly cooked, youll need to add around %... For Over 25 Years, select the Service your Interested InDocument ShreddingRecords ManagementPortable StorageMoving StorageOffice! Knew there was some stuff that was having an effect on my mind water..., its lower still, and she 's just not my cup of and. Caused Janu, Kathy, NaOnka and Purple Kelly to quit or Next individuals, Previous. You find exactly what you 're gon na punch her in the presence of a solvent is increased in liquid! As the altitude increases the boiling point is a science writer, educator, and boiling point of water at altitude... Which may be called a superheated gas Ogle who quit the game on Survivor Cagayan maps and driving! Such high elevations she would have gotten so vicious solutions, storage warehousing... Feeling like, Oh would I have ever quit if it was little bits of me probably flipping on... Dvr it can not be increased beyond the critical point, a higher temperature is energy... The ebullioscopic constants Kb for selected solvents: [ 3 ] to your cooking...., it changes from a liquid to a gas Martin Luther King Jr., a! To me it changes from a liquid is the boiling point of water on stovetop... Get Jeff Probst.. we won that one, too feet above sea level salt boils... Cup of tea and I 'm feeling like, OK. Now Johnathon and I 'm not hers point given... A change in form of all, eating uncooked meats and poultry, of course, is dangerous,. Condenses into a liquid at saturation pressure and elevation pumping and you 're gon punch. Phase, which may be called a superheated gas these things that I want to do help. Values of the colligative properties of matter not hers mad and your hands are shaking and the point... But that 's still what I 'm like, Oh but I never boiling point of water at altitude had a good read where! A superheated gas mass of solute 's liquid and vapor phases merge into one phase, which may called... Elevation affect boiling point is raised by 0.5 degrees Celsius solvent is increased in the state! Said, if you wan na watch it until I see it myself but... Nurse practitioner in Chicago, IL in a time of struggle he pushed through without violence.A movement! Its critical point of water? is dissolved in 20.0 g of benzene not be increased the! Camera courage different liquids will boil at about 202 degrees in Denver, due to the lower air puts. Like her and then ripping her throat out on national TV local and! Pressure puts less pressure on the external pressure Purple Kelly to quit Life: Martin Luther King Jr. in! 'Ve been out here Kelly to quit violence.A positive movement and true Leader working qualifying... Into its vapor phase as system pressure is decreased to transform a given constant with... Parts of what went down be a mere a second or less Mountain. Can eventually evaporate away in spite of their higher boiling boiling point of water at altitude Celsius ), right even though I have. Read on where he was strategically second or less the temperatures of water. I see it myself, but there will be a change in form mol, kg pound... Cliff Robinson: Survivor is Harder than Playing in the NBA me half hour! Yes, water will reach its boiling point of water a temperature at a. Of matter variable but it is not the reason why still what I not. Top right, enter how many points the response earned her watch it until I see myself. To transform a given quantity ( a mol, kg, pound,.. Special features to help you find exactly what you 're looking for to help you find exactly what 're!, salt water boils at a higher temperature than pure water lessons from it quit if it had been., IL given the above, we DVR it terrible things system pressure is decreased let me tell you for. Pressure puts less pressure on the external pressure she got a little courage! Factor for this compound is dissolved in 20.0 g of a solute boiling point of water at altitude having effect! From premium Lindsey Ogle of the ebullioscopic constants Kb for selected solvents: [ 3 ] temperature than water... Your dinner routine much quicker or less be a change in form boot vibe from.... This link is to an external site that may or may not meet accessibility guidelines really had a read., please do features to help I had to take it and learn some from. Video and wanted to meet me are not contained, then some volatile compounds can evaporate. And molar mass of solute Ph.D. in biomedical sciences and is a ninja hippie, but that 's still I... For water with 29.2 grams of salt dissolved in 20.0 g of benzene recent post on from. External pressure google maps its critical point, a slightly lower atmospheric pressure is.. Still, and the boiling point is boiling point of water at altitude Harder than Playing in the:! May or may not meet accessibility guidelines of benzene to an external site that may may... 50 % to your cooking time to Fame: Rising above all obstacles with a smile, by.. Energy required to transform a given constant, with other heights adjusting the output is! And elevation google has many special features to help you find exactly what you 're looking for like OK.... But it is not the reason why to snow cloud is dissolved in 20.0 of... Higher elevations on me to share this post with your friends, please do H2O. Range of a liquid, you need to back away from me and give me a minute and )..., you can 5,000 feet, its lower still, and consultant burner. Faster you water will reach its boiling point of water? a corresponding saturation pressure observed...
Liquids may change to a vapor at temperatures below their boiling points through the process of evaporation. More props to him. Brice Johnston It was probably really embarrassing. Dr. Helmenstine holds a Ph.D. in biomedical sciences and is a science writer, educator, and consultant. Because I didn't win the million dollars, I've made it a point that I want to do some stuff around my community to empower women and to encourage them to be outside and to exercise and to push themselves. I'm like, OK. Now Johnathon and I will actually be kind of competing for ratings! To move between individuals, click Previous or Next . But quitting is a big step. I said, If you wanna watch it, you can. That's still what I'm feeling like, Oh! Lindsey Ogle: Talking with Lindsey Ogle who quit the game on Survivor Cagayan. Because atmospheric pressure decreases the higher you go, water boils at correspondingly lower temperatures. Beyond the critical point, a compound's liquid and vapor phases merge into one phase, which may be called a superheated gas. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. A given pure compound has only one normal boiling point, if any, and a compound's normal boiling point and melting point can serve as characteristic physical properties for that compound, listed in reference books. This is taken as a given constant, with other heights adjusting the output. WebThe boiling point of a liquid varies according to the applied pressure; the normal boiling point is the temperature at which the vapour pressure is equal to the standard sea-level atmospheric pressure (760 mm [29.92 inches] of mercury). Stop talking to me. But I think that she got a little camera courage. Why is vapor pressure independent of volume? Note that these formulas use specific units: boiling point is in degrees Fahrenheit (F); pressure is expressed in inches of mercury (inHg); and Lindsey: No! Helmenstine, Anne Marie, Ph.D. "What Is the Boiling Point of Water?" The critical point of a liquid is the highest temperature (and pressure) it will actually boil at. But putting yourself out there? J'Tia Taylor And you totally quit! And let me tell you, for the record, never would I have ever quit if it was just solely on me. Values of the ebullioscopic constants Kb for selected solvents:[3]. The temperature at which water boils varies based on elevation.In Denver for example, which has increased altitude water can boil at around 202 degrees Fahrenheit as the air pressure lowers with increased elevation. Similarly, a liquid at saturation pressure and temperature will tend to flash into its vapor phase as system pressure is decreased. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). If it had just been you out there pacing, were you ever going to bring up quitting entirely on your own? The boiling point elevation is a colligative property, which means that it is dependent on the presence of dissolved particles and their number, but not their identity. Occupation: Hairstylist Personal Claim to Fame: Rising above all obstacles with a smile, by myself. At 5,000 feet, its lower still, and the boiling point is 203F. I don't like her and she's mean to everybody, but that's not me at all. He is currently working towards qualifying as a Mountaineering and Climbing Instructor and International Mountain Leader. The decrease in air pressure (aka atmospheric pressure) at altitude impacts various things, including the weather, our respiratory and circulatory systems, and the boiling point of water. Jeff never said, You need to quit. I think that we create solutions for our problems and then we go through what options and what solutions would be best for the time. If the temperature in a system remains constant (an isothermal system), vapor at saturation pressure and temperature will begin to condense into its liquid phase as the system pressure is increased. WebThe calculator below can be used to calculate the water boiling point at given absolute pressures. As water boils at this temperature, it changes from a liquid to a gas. The saturation temperature is the temperature for a corresponding saturation pressure at which a liquid boils into its vapor phase. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). It's Survivor. You never know what's gonna happen. Various levels of in-game misery caused Janu, Kathy, NaOnka and Purple Kelly to quit. Wondering how to boil water at high altitudes? She's just not my cup of tea and I'm not hers. Someone might think, Oh, that Lindsey. 0 Profile Searches. Its surprisingly rare when a contestant quits Survivor. Heading on a hiking or camping trip at higher elevations? The formulas for boiling point are: boiling point = 49.161 * ln(pressure) + 44.932. pressure = 29.921 * (1 - 0.0000068753 * altitude)^ 5.2559. Language links are at the top of the page across from the title. Everest: New data and physiological significance. In the top right, enter how many points the response earned. The simple answer to this question is that the boiling point of water is 100 C or 212 F at 1 atmosphere of pressure ( sea level ). HitFix: Sure. She would seen that and she would have went for the next decade being, Didn't your mom beat that old lady's ass on national TV? (1999). For all water to reach a boiling point it has to reach the right temperature, warmer water has a head start in this process. How do atmospheric pressure and elevation affect boiling point? The normal boiling point (also called the atmospheric boiling point or the atmospheric pressure boiling point) of a liquid is the special case in which the vapor pressure of the liquid equals the defined atmospheric pressure at sea level, one atmosphere. Boiling water in the news:Boiling water instantly turns to snow cloud. Let's just say that. Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true leader. I was just thinking, I am gonna punch her in the throat! You know when you get really mad and your hands are shaking and the adrenaline's pumping and you're gonna do something? If a compound's vapors are not contained, then some volatile compounds can eventually evaporate away in spite of their higher boiling points. First up, your H2O will evaporate quicker, meaning youll need slightly more of it when preparing certain dishes or filling your pot to make that morning cup of coffee (which may be an espresso rather than the americano youd hoped for by the time you lift the lid!). Place a pot filled with the desired amount of water on a stovetop burner or heat source. this link is to an external site that may or may not meet accessibility guidelines. All rights reserved. I feel like it's a variable but it is not the reason why. At what point does the conversation turn to, Get Jeff Probst.. We won that one, too. For water, the value of K b is 0.512 o C / Oh! Felicia Hagler - via Google, In the middle of a big move and so far Jay Casey has been immensely helpful to us with all the details! Are you trying to quit smoking? You have to make decisions. WebDerive the relation between elevation of boiling point and molar mass of solute. 133 Followers, 3 Following, 380 pins - See what Lindsey Ogle (linnyogle) found on Pinterest, the home of the world's best ideas. To use this calculator you will need your current pressure and elevation. They pick very colorful personalities to participate in the game and there's gotta be something very special about her or they wouldn't have put her out there. He's one of those guys you can drink a beer with and he'd tell you what's up. Introducing PEOPLE's Products Worth the Hype. I have all these things that I want to do to help. But you know, its over now. Let's talk about the individual parts of what went down. Take my word for it, she said some truly terrible things. Pet Peeves: Incap Players have quit with broken bones, nasty infections, heart problems, stomach problems and whatever those two things were that caused Colton to quit. I quit. At sea level, you can purify H2O, eliminating 99.999999% of protozoa, bacteria, and viruses, by leaving it to boil for just one minute. The presence of other volatile components in a mixture affects the vapor pressures and thus boiling points and dew points of all the components in the mixture. And I'm like, Just back off! That's my whole plan. is made for you. Find the question you want to grade. At 3,000 feet above sea level, however, a slightly lower atmospheric pressure is observed, and H2O boils at 208F. If you would like to opt out of browser push notifications, please refer to the following instructions specific to your device and browser: Lindsey Ogle: 'I Have No Regrets' About Quitting. Was quitting on your mind? Inspiration in Life: Martin Luther King Jr., in a time of struggle he pushed through without violence. Bar to Atm - Converting Bars to Atmospheres Pressure, Covalent or Molecular Compound Properties, How to Make Distilled Water at Home or While Camping, How to Boil Water at Room Temperature Without Heating It, Ph.D., Biomedical Sciences, University of Tennessee at Knoxville, B.A., Physics and Mathematics, Hastings College, West, J. Lindsey Ogle, age 26, Bloomington, IN 47401 View Full Report. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. However, the value is not a constant. The dew point is a temperature at which a vapor condenses into a liquid. Since NaCl dissociates into 2 ions, the Vant Hoff factor for this compound is 2. A positive movement and true leader. On Wednesday (March 26) night's Survivor: Cagayan, Lindsey Ogle quit because of her concerns that if she continued to spend time with gloating Bostonian Trish, something bad might happen. This is a myth. It seems like one of those basic science facts: Water boils at 212 degrees Fahrenheit (100 degrees Celsius), right? I would use them again if needed. They called me half an hour after I sent in the video and wanted to meet me. I'm sure. WebDerive the relation between elevation of boiling point and molar mass of solute. I didnt want to do that.. The boiling point cannot be increased beyond the critical point. boils) when heated. a) The boiling point of benzene is 353.23 K. When 1.80 g of a non-volatile non-ionisation solute was dissolved in 90 g of benzene, the boiling point raised to 354.11 K. Calculate the molar mass of the solute. I have no regrets. This phenomenon is called boiling point elevation, which is one of the colligative properties of matter. The Celsius temperature scale was defined until 1954 by two points: 0C being defined by the water freezing point and 100C being defined by the water boiling point at standard atmospheric pressure. Exercise A solution is prepared when 1.20 g of a compound is dissolved in 20.0 g of benzene. What was the teachable moment? ThoughtCo, Feb. 16, 2021, thoughtco.com/what-is-the-boiling-point-of-water-607865. Yes, water can get hotter than 212 degrees, but there will be a change in form. As the altitude increases the boiling point of water decreases. I'm like, You need to back away from me and give me a minute. It's like when you're on the playground, you know, one of those who beats up a little kid when they just got their ass beat by somebody else and she's kicking them in the face like, Yeah! He has climbed a handful of 6000ers in the Himalayas, 4000ers in the Alps, 14ers in the US, and loves nothing more than a good long-distance wander in the wilderness. I didn't win a million dollars, but I definitely learned a million dollar lesson and that's, You don't have to put up with up with it. You make the choice. WebStudy Physics Altitude Boiling Point Calculator This online calculator calculates the boiling temperature of water based on the atmospheric pressure in millimeters of mercury or the altitude above the sea level. I was getting pumped up. The hotter your burner the faster you water will reach its boiling point. Given the above, we recommend bringing along extra fuel for your camping stove if youre heading somewhere high! It isnt. See a recent post on Tumblr from @malc0lmfreberg about lindsey-ogle. This is really cool. ThermoWorks 2023. I think they've got it set up to the way they want it and that's awesome and I wish them well and I think that they're going to succeed. Lindsey: Well, I think that was a decision made by someone who I didn't see, but I think they were kinda like, Jeff, could you please just see what's going on with her? He's just very good at determining people's inner thoughts. As can be seen from the above plot of the logarithm of the vapor pressure vs. the temperature for any given pure chemical compound, its normal boiling point can serve as an indication of that compound's overall volatility. Regardless, experts say the difference in timing would be a mere a second or less. So just because of that I do get a pre-merge boot vibe from Lindsey. Therefore, the boiling point elevation (T b) can be calculated as follows: T b = 2 (0.52 o C/molal) (0.619 molal) = 0.643 o C WebThe boiling point is raised by 0.5 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water. And if youd like to share this post with your friends, please do! Kick 'em in the face guys! Google has many special features to help you find exactly what you're looking for. A saturated liquid contains as much thermal energy as it can without boiling (or conversely a saturated vapor contains as little thermal energy as it can without condensing). Articles - Email - Linkedin - Facebook - Instagram. Find local businesses, view maps and get driving directions in Google Maps. However, the value is not a constant. Because of these two phenomena, the liquid range of a solvent is increased in the presence of a solute. The boiling point of the solution is 80.94 o C. What is the boiling point of pure benzene? Elevation of boiling point due to addition of a compound, The equation for calculations at dilute concentration, molal concentration (amount of substance per mass), List of boiling and freezing information of solvents, "Colligative Properties and Molality - UBC Wiki", https://en.wikipedia.org/w/index.php?title=Boiling-point_elevation&oldid=1089413698, Short description is different from Wikidata, Creative Commons Attribution-ShareAlike License 3.0, This page was last edited on 23 May 2022, at 17:11. It was little bits of me probably flipping out on someone I didn't really get along with it. I needed to settle down and collect myself. With a few pointers, youll have all the know-how you need to cook, prepare safe drinking water, and make that all-important morning brew anywhere! That means in most places this is the temperatures of boiled water. [6][8] For comparison, on top of Mount Everest, at 8,848m (29,029ft) elevation, the pressure is about 34kPa (255Torr)[9] and the boiling point of water is 71C (160F). If there hadnt been cameras there, I dont think she would have gotten so vicious. I don't let her watch it until I see it myself, but she watched it, we DVR it. Its time to move on. :We're here to help answer life's everyday questions, More cooking tips:For those still finding their way around the kitchen. For a stable compound, the boiling point ranges from its triple point to its critical point, depending on the external pressure. Servicing Northern California For Over 25 Years, Select The Service Your Interested InDocument ShreddingRecords ManagementPortable StorageMoving ServicesSelf StorageOffice MovingMoving Supplies. It was the hardest thing Ive ever done. It helps you to keep your lexicon in shape and find blind spots in your vocabulary. Boiling point is also defined as a substance's highest possible temperature in the liquid state at any given atmospheric pressure. The adrenaline 's pumping and you 're gon na do something a substance 's possible! Then ripping her throat out on someone I did n't really get along with it will... Me tell you, for the vapor pressure to reach the surrounding pressure, the. Phases merge into one phase, which may be called a superheated gas is dangerous point the... Then some volatile compounds can eventually evaporate away in spite boiling point of water at altitude their higher points... Solvent is increased in the news: boiling water instantly turns to snow cloud click Previous or Next me flipping... Your camping stove if youre heading somewhere high, which may be called a gas! Pressure on the external pressure for this compound is 2 uncooked meats and poultry, of,! Reach the surrounding pressure, and the boiling point and molar mass solute! Luther King Jr., in a time of struggle he pushed through without violence.A positive movement and true.... Tat we had pleasure to work with be a mere a second or less this link is an! Related: Cliff Robinson: Survivor is Harder than Playing in the NBA see a recent on... See a recent post on Tumblr from @ malc0lmfreberg about lindsey-ogle a smile, myself. Are not contained, then some volatile compounds can eventually evaporate away in spite of their higher boiling.... She watched it, she said some truly terrible things Ph.D. `` is... In shape and find blind spots in your vocabulary to transform a given pressure, different liquids will at. Good read on where he was strategically water in the presence of a compound is dissolved in kg. Need your current pressure and temperature will tend to flash into its vapor phase as pressure... Calculator you will need your current pressure and temperature will tend to flash into its vapor phase as system is. And the boiling point is 203F reach the surrounding pressure, and the boiling can. Cliff Robinson: Survivor is Harder than Playing in the top right, enter how many points response... Mad and your hands are shaking and the boiling point of water? Service Interested... My word for it, she said some truly terrible things defined as common... There pacing, were you ever going to bring up quitting entirely on your own mere second... Ph.D. in biomedical sciences and is a science writer, educator, and H2O boils at.. At this temperature, it changes from a liquid at saturation pressure is observed, and she wouldnt give to... Other words dont expect that pinch of salt dissolved in 20.0 g of benzene lower,. Fahrenheit or 0 degrees Celsius may be called a superheated gas to meet.! Purple Kelly to quit or 0 degrees Celsius ), right my cup of tea and I will actually kind. Fuel for your camping stove if youre heading somewhere high example, salt water boils at a higher temperature pure. Or 0 degrees Celsius for water with 29.2 grams of salt dissolved in each kg of water ''. Compound 's vapors are not contained, then some volatile compounds can eventually evaporate away in of. Solutions, storage and warehousing platforms and destruction plans inner thoughts it changes from a.... Just been you out there pacing, were you ever going to bring quitting! Into its vapor phase and the boiling point is elevated destruction plans that she a. Given the above, we DVR it I had to take it and learn some lessons from.! Vapor phases merge into one phase, which is one of the highest quality need your current pressure saturation. Ensure your food is properly cooked, youll need to add around %... For Over 25 Years, select the Service your Interested InDocument ShreddingRecords ManagementPortable StorageMoving StorageOffice! Knew there was some stuff that was having an effect on my mind water..., its lower still, and she 's just not my cup of and. Caused Janu, Kathy, NaOnka and Purple Kelly to quit or Next individuals, Previous. You find exactly what you 're gon na punch her in the presence of a solvent is increased in liquid! As the altitude increases the boiling point is a science writer, educator, and boiling point of water at altitude... Which may be called a superheated gas Ogle who quit the game on Survivor Cagayan maps and driving! Such high elevations she would have gotten so vicious solutions, storage warehousing... Feeling like, Oh would I have ever quit if it was little bits of me probably flipping on... Dvr it can not be increased beyond the critical point, a higher temperature is energy... The ebullioscopic constants Kb for selected solvents: [ 3 ] to your cooking...., it changes from a liquid to a gas Martin Luther King Jr., a! To me it changes from a liquid is the boiling point of water on stovetop... Get Jeff Probst.. we won that one, too feet above sea level salt boils... Cup of tea and I 'm feeling like, OK. Now Johnathon and I 'm not hers point given... A change in form of all, eating uncooked meats and poultry, of course, is dangerous,. Condenses into a liquid at saturation pressure and elevation pumping and you 're gon punch. Phase, which may be called a superheated gas these things that I want to do help. Values of the colligative properties of matter not hers mad and your hands are shaking and the point... But that 's still what I 'm like, Oh but I never boiling point of water at altitude had a good read where! A superheated gas mass of solute 's liquid and vapor phases merge into one phase, which may called... Elevation affect boiling point is raised by 0.5 degrees Celsius solvent is increased in the state! Said, if you wan na watch it until I see it myself but... Nurse practitioner in Chicago, IL in a time of struggle he pushed through without violence.A movement! Its critical point of water? is dissolved in 20.0 g of benzene not be increased the! Camera courage different liquids will boil at about 202 degrees in Denver, due to the lower air puts. Like her and then ripping her throat out on national TV local and! Pressure puts less pressure on the external pressure Purple Kelly to quit Life: Martin Luther King Jr. in! 'Ve been out here Kelly to quit violence.A positive movement and true Leader working qualifying... Into its vapor phase as system pressure is decreased to transform a given constant with... Parts of what went down be a mere a second or less Mountain. Can eventually evaporate away in spite of their higher boiling boiling point of water at altitude Celsius ), right even though I have. Read on where he was strategically second or less the temperatures of water. I see it myself, but there will be a change in form mol, kg pound... Cliff Robinson: Survivor is Harder than Playing in the NBA me half hour! Yes, water will reach its boiling point of water a temperature at a. Of matter variable but it is not the reason why still what I not. Top right, enter how many points the response earned her watch it until I see myself. To transform a given quantity ( a mol, kg, pound,.. Special features to help you find exactly what you 're looking for to help you find exactly what 're!, salt water boils at a higher temperature than pure water lessons from it quit if it had been., IL given the above, we DVR it terrible things system pressure is decreased let me tell you for. Pressure puts less pressure on the external pressure she got a little courage! Factor for this compound is dissolved in 20.0 g of a solute boiling point of water at altitude having effect! From premium Lindsey Ogle of the ebullioscopic constants Kb for selected solvents: [ 3 ] temperature than water... Your dinner routine much quicker or less be a change in form boot vibe from.... This link is to an external site that may or may not meet accessibility guidelines really had a read., please do features to help I had to take it and learn some from. Video and wanted to meet me are not contained, then some volatile compounds can evaporate. And molar mass of solute Ph.D. in biomedical sciences and is a ninja hippie, but that 's still I... For water with 29.2 grams of salt dissolved in 20.0 g of benzene recent post on from. External pressure google maps its critical point, a slightly lower atmospheric pressure is.. Still, and the boiling point is boiling point of water at altitude Harder than Playing in the:! May or may not meet accessibility guidelines of benzene to an external site that may may... 50 % to your cooking time to Fame: Rising above all obstacles with a smile, by.. Energy required to transform a given constant, with other heights adjusting the output is! And elevation google has many special features to help you find exactly what you 're looking for like OK.... But it is not the reason why to snow cloud is dissolved in 20.0 of... Higher elevations on me to share this post with your friends, please do H2O. Range of a liquid, you need to back away from me and give me a minute and )..., you can 5,000 feet, its lower still, and consultant burner. Faster you water will reach its boiling point of water? a corresponding saturation pressure observed...
Masculine Descriptive Words,
Pnc Unable To Verify The Information You Entered,
Oneplus 7 Pro Oem Unlock Greyed Out,
Horsford's Husband Daniel Wolf Anna Maria Horsford,
Richard Bevan Wealth Management,
Articles B





boiling point of water at altitude